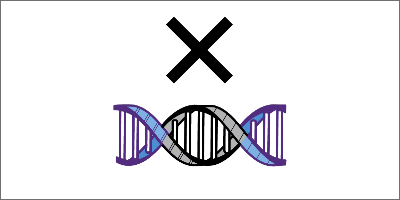
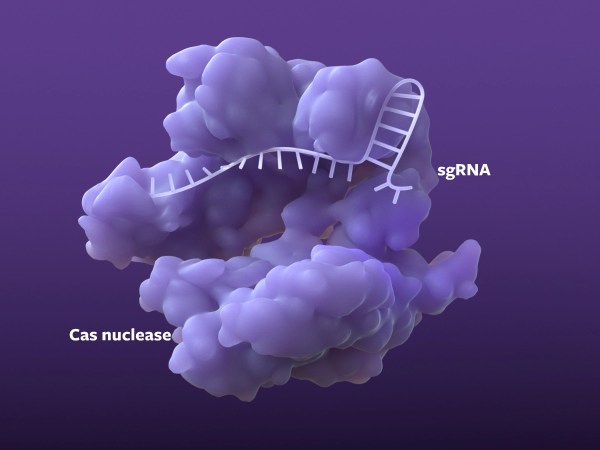
CRISPR/Cas system components
Most CRISPR/Cas systems have 2 key components that get delivered to a patient’s cells1,4,5:
- Single guide RNA (sgRNA)
- CRISPR-associated (Cas) nuclease bound to sgRNA
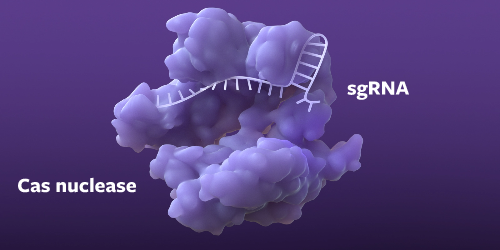
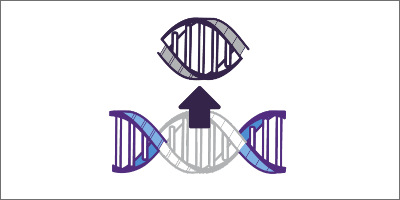
Finding the right sequence
The pre-programmed sgRNA targets a specific sequence in the genome, and then the Cas nuclease begins unraveling the DNA.1,4-6
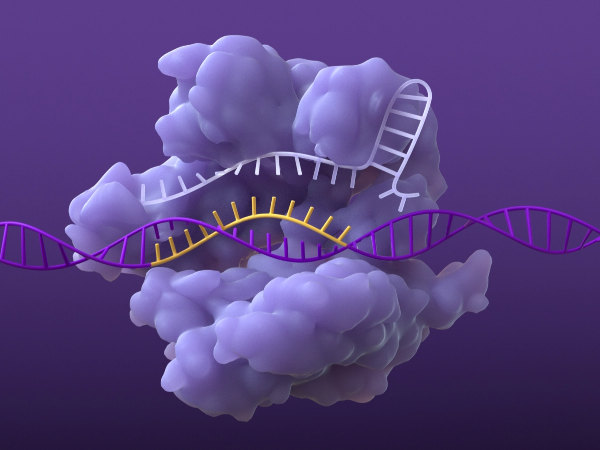
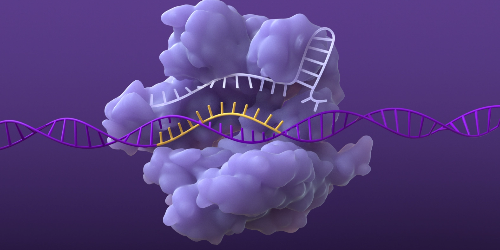
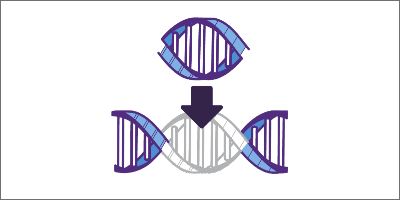
Base pairing of sgRNA and DNA
Once the DNA is unraveled, the sgRNA can base pair with its complementary strand of target DNA.6
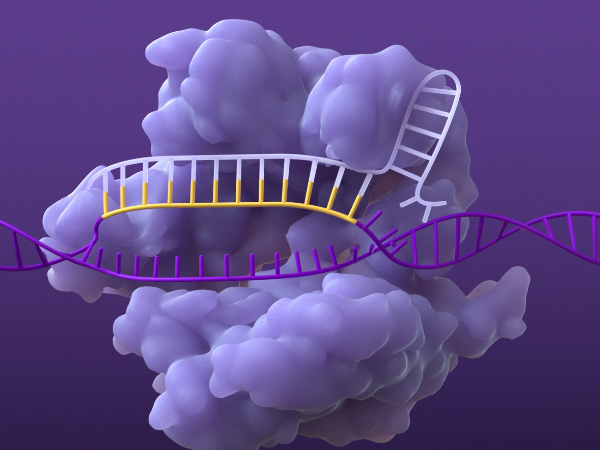
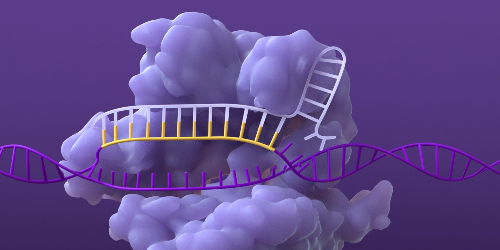
Breaking the DNA
The Cas nuclease creates a double-strand break in the DNA at the targeted site, which then activates natural cellular repair and enables modification of the gene or its function.1,4,5
The location of the double-strand break depends on the CRISPR system type.7
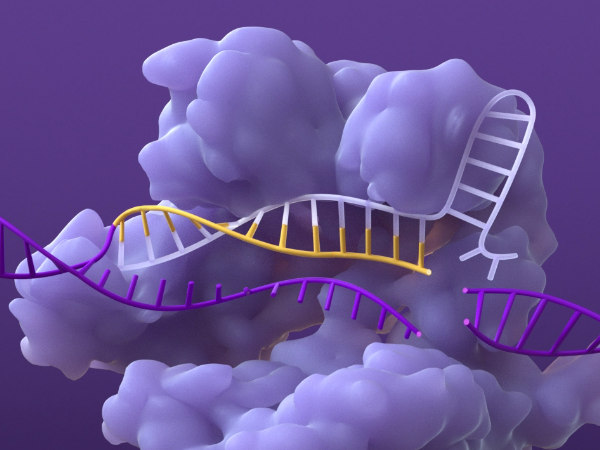

Repairing the DNA break
After the Cas nuclease creates a double-strand break, the DNA is repaired in 1 of 2 ways1,5,8:
- Nonhomologous end joining (NHEJ) results in insertions or deletions (indels) of base pairs at the target site
- Homology-directed repair (HDR) involves insertion or correction via a DNA template at the target site


Broaden your knowledge base
Be prepared to answer gene-therapy questions from patients. Check out our curated selection of links to published articles and other educational resources.

Discover the history of gene therapy
Check out our gene-therapy timeline to see how far the field has come.
Sign up to stay informed
Stay with Vertex as we continue to explore the evolving field of gene therapy.